African Swine Fever Virus Real-time PCR Test Kit(Lyophilized Microspheres)
African Swine Fever Virus Real-time PCR Test Kit(Lyophilized Microspheres)
African Swine Fever Virus Real-time PCR Test Kit(Lyophilized Microspheres) is used for detecting the DNA of African Swine Fever Virus (ASFV) in pig blood, serum, plasma, tissues and swab samples. The test results can be used for the auxiliary diagnosis of African Swine Fever Virus infection.
Specification
Specification
48 Tests / box
Precautions
Precautions
- The test results of this kit are only a diagnostic aid. Please read this manual carefully before use.
- This kit is suitable for ASFV virus detection only.
- Before the experiment, please be familiar with and master the operation methods and matters needing attention of various instruments.
Storage
Storage
The reagents should be stored in a light-proof and air-tight manner at room temperature. The shelf life of the kit is 12 months.
Validity Period
Validity Period
The shelf life of the kit is 12 months.
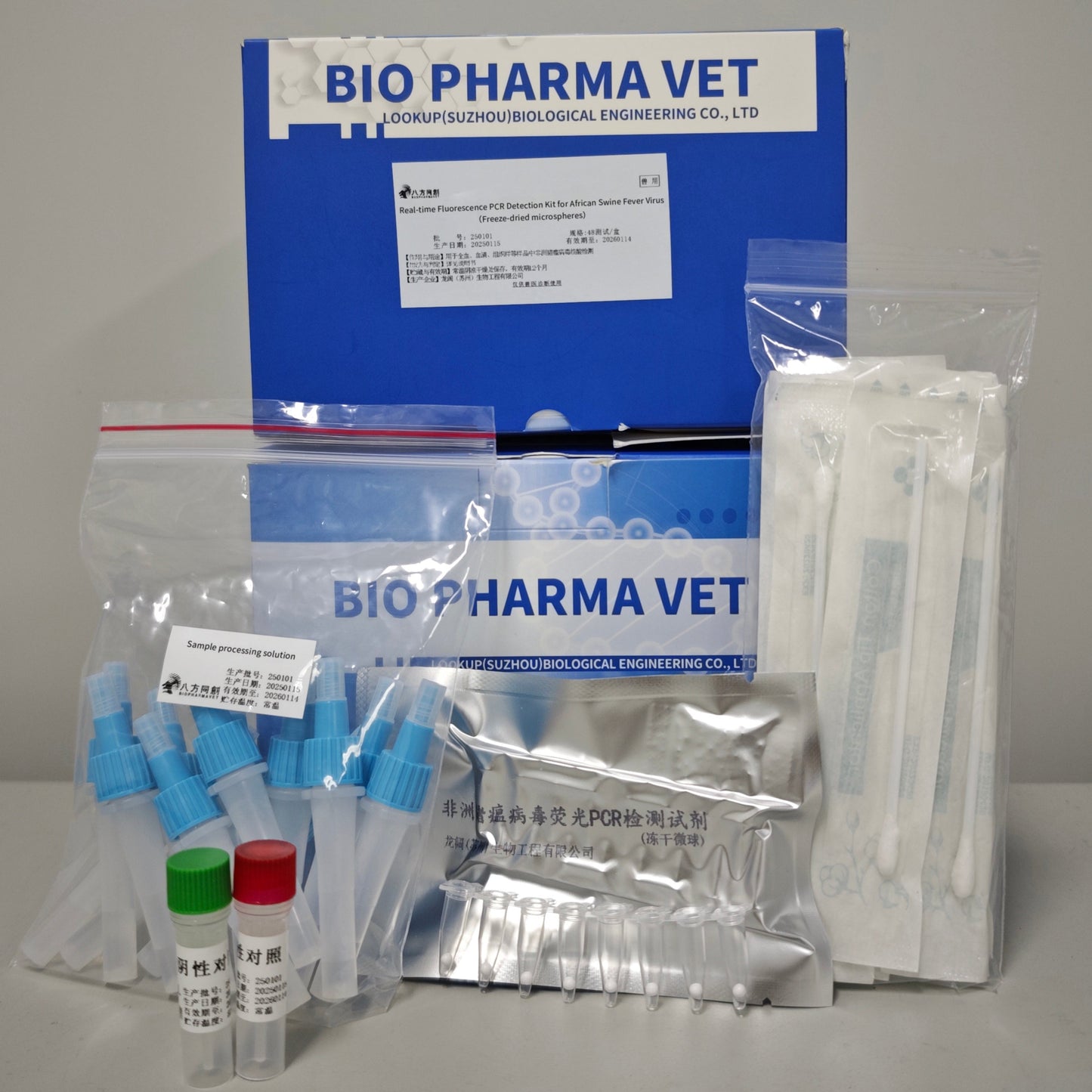
Product Information(Real-time fluorescence PCR detection kit for African swine fever virus)
This product is for scientific research purposes. It contains reaction buffer, DNA polymerase, UDG enzyme, lyophilization protectant, and primer-probe components. The reaction buffer contains essential components for amplification, such as Mg2+ and dNTP. This product is in lyophilized formulation.
| Reagent Name | Specification and Loading |
| Real-time fluorescence PCR detection reagent for African swine fever virus (Lyophilized Microspheres) | 16 tests per strip, 3 strip per box |
| negative control | 100 μ L / tube, 1 tube / box |
| positive control | 100 μ L / tube, 1 tube / box |
| Sample treatment solution | 450 μ L / tube, 48 tubes / box |
| Disposable cotton swabs | 24 pieces per box |
| Disposable dropper | 24 pieces per box |
Remarks:
- Components of products with different batch numbers shall not be mixed or interchanged.
- Equipment and reagents required for the test: centrifuge; fluorescence quantitative PCR system.
Sample Requirements
- Applicable sample types: serum, whole blood, swabs, etc.
- Sample pretreatment:
2.1 Saliva, nasal and oral fluids, anal swabs, and environmental samples: Collect samples such as saliva, nasal and oral fluids, anal swabs of the pigs to be tested or environmental samples with a disposable cotton swab, and directly put them into the sample processing solution tube. Rotate the tube 10 times to mix well, and let it stand for 1 minute to ensure that the nucleic acid of the samples is fully lysed in the sample processing solution.
2.2 Serum, whole blood: Use a disposable dropper to pipette the whole blood or serum sample of the pig to be tested, add 1 drop (15μL) into the sample processing solution tube. Mix thoroughly, then let it stand for 1 minute to ensure that the nucleic acid of the sample is fully lysed in the sample processing solution.
Testing Methods
- Reagent Preparation Calculate the number N of reaction tubes of the African Swine Fever Virus Real-time PCR Test Kit (Lyophilized Microspheres)
- Sample Loading
2.1 Add 25μL of the processed samples into the reaction tubes containing the African Swine Fever Virus Real-time Test Kit (Lyophilized Microspheres).
2.2 Add 20μL of paraffin oil into all the reaction tubes (If the fluorescence PCR instrument is equipped with a heated lid, there is no need to perform this step.) and perform an instantaneous centrifugation.
3. PCR Amplification
3.1 Add 1 drop (25μL) of each processed sample to be tested into the reaction tube in sequence.
3.2 Add 1 drop (20μL) of paraffin oil into the reaction tube, close the tube cap tightly, and centrifuge briefly.
3.3 Setting of cycling parameters
| Step | Conventional Procedure | |||
| temperature | Time | Cycles | ||
| 1 | UNG Enzyme Reaction | 50℃ | 2min | 1 |
| 2 | Taq Enzyme Activation | 95℃ | 2min | 1 |
| 3 | Denaturation | 95℃ | 15s | 45 |
| Annealing, Extension, Fluorescence Collection | 60℃ | 30s | ||
| 4 | Instrument Cooling | 25℃ | 10s | 1 |
4. Quality Control
4.1 ASFV-Negative Control: There should be no Ct value displayed in the FAM channel, and the Ct value in the ROX channel should be ≤ 35.
4.2 ASFV-Positive Control: The Ct value in the FAM channel should be ≤ 35, and the Ct value in the ROX channel should be ≤ 35.
4.3 The above requirements must be met simultaneously in the same experiment. Otherwise, the current experiment is invalid and needs to be repeated.
5. Result Judgement
5.1 If the Ct value of the ROX channel for sample detection is ≤ 35 and the amplification curve is a typical S-shaped curve, then the result can be judged according to "5.2 - 5.3".
5.2 If the Ct value of the FAM channel is measured to be ≤ 45 and the amplification curve is a typical S-shaped curve, it should be reported as ASFV nucleic acid positive.
5.3 If there is no Ct value in the FAM channel or there is no typical S-shaped amplification curve, it should be reported as ASFV nucleic acid negative.
Precautions
1. The test results of this kit are only for diagnostic assistance. Please read this instruction manual carefully before using it.
2.This kit is only applicable for the detection of African Swine Fever Virus (ASFV).
3.Please familiarize yourself with the operation methods and precautions of all the instruments to be used before conducting the experiment.
4.After adding the sample, the sample processing solution should be as color - less and transparent as possible. Otherwise, it may affect the test results.
5.When adding the sample processing solution, do it slowly to avoid the formation of air bubbles as much as possible. After opening, the African Swine Fever Virus Fluorescent PCR Detection Reagent (Lyophilized Microspheres) should be stored in a sealed container at room temperature.


